We have been focused on determining the function of sleep and the cause of narcolepsy. We have investigated the roles of the peptide hypocretin (also called Hcrt or orexin) and of the locus coeruleus (a brainstem nucleus containing neurons that release norepinephrine), in narcolepsy. Our work includes postmortem immunohistochemistry of brains willed by people with narcolepsy and by controls, as well as observations of hypocretin and norepinephrine release in living, non-narcoleptic humans and in narcoleptic dogs.
We were the first to report the loss of hypothalamic hypocretin neurons in people with narcolepsy (see below-left, submitted to Society for Neuroscience March 5, 2000 and [PMID:11055430]), the first to record the activity of hypocretin neurons in normal animals (rats) (in 2005 [PMID:15924864]), finding that these neurons fired in relation to approach/pleasurable behavior (click here to see video) and the first to record hypocretin release in the non-narcoleptic human brain (in 2013 [PMID:23462990]), finding greatly elevated hypocretin release during pleasurable social interactions and minimal release during aversion, disappointment or pain. We were also the first and only ones to record neuronal activity in narcolepsy, showing that locus coeruleus neurons cease firing during cataplexy in narcoleptic dogs, just as they cease firing during the muscle atonia of REM sleep [PMID:10391445], This latter finding led to our discovery, 25 years later, of the loss of locus coeruleus neurons in human narcolepsy (see "In 2025 . . ." below).
Five to thirty percent of people diagnosed with "typical narcolepsy with cataplexy" have absolutely normal levels of cerebrospinal hypocretin ([PMID: 10615891]; [PMID: 12374492]; [PMID: 17702265]; [PMID 33539807]; [PMID: 26564387]; [PMID: 32406370]; [PMID: 22942503]; [PMID: 16006155], [PMID: 15081654]). This finding is not consistent with the popular hypothesis that the loss of hypocretin causes sleepiness and cataplexy, the principal symptoms of narcolepsy. The cerebrospinal hypocretin levels in people diagnosed with "narcolepsy without cataplexy," the majority of people diagnosed with narcolepsy, do not differ from hypocretin levels in normal controls. ([PMID: 17702265], [PMID: 24882898], [PMID:21102568]). Again, this is not consistent with the hypothesis that hypocretin loss causes narcolepsy. Neither the activity of hypocretin neurons in rats (see blue video link above), nor hypocretin release in non-narcoleptic humans [PMID:23462990] are strongly correlated with alertness (EEG activation) or with muscle tone, the two parameters that are altered in narcolepsy. Because of the relation of hypocretin neuronal discharge to pleasurable activities, it is reasonable to hypothesize that the loss of hypocretin neurons may be responsible for the increased incidence of depression in people with narcolepsy, but the main symptoms of narcolepsy cannot be explained by the loss of hypocretin neurons ([PMID: 15924864], [PMID: 22031892], [PMID 23462990],[PMID: 40184220]). However, consistent with hypocretin's relation to pleasure, hypocretin has a major role in opioid addiction (see the last paragraph).
In 2025, we discovered that all people that had narcolepsy with cataplexy had a loss of locus coeruleus norepinephrine neurons (Chi squared, p<0.0001) [doi.org/10.1101/2025.04.12.648456])(now in press in Nature Communications). Locus coeruleus neurons have descending axons that activate motoneurons and we find that their discharge cessation is tightly correlated with the loss of muscle tone loss in REM sleep and with the loss of muscle tone loss in cataplexy ([PMID:10391445], [PMID:21102568], [PMID:11069963], [PMID:11353017], [PMID: 37155728], [PMID: 31174102]. Thus, the loss of locus coeruleus neurons can explain cataplexy. These norepinephrine neurons also have ascending axons and their loss decreases alertness and increases sleepiness ([PMID:11549748], [PMID:20668280]). Therefore the loss of locus coeruleus neurons can explain the two main symptoms of narcolepsy. Our recent study [doi.org/10.1101/2025.04.12.648456] also shows that the loss of locus coeruleus neurons is not caused by the loss of hypocretin neurons. Consistent with our finding of a major loss of locus coeruleus neurons in narcolepsy, clinical trials have shown that Reboxetine, a selective norepinephrine reuptake inhibitor, available in Europe but not in the US, produces significant reductions in cataplexy and improvements in excessive daytime sleepiness and cognitive function in narcolepsy with cataplexy [PMID: 11322710]. In a similar manner, Solriamfetol, a dopamine and norepinephrine reuptake inhibitor, produces a significant reduction in cataplexy and excessive daytime sleepiness. Alpha-1 adrenergic agonists are known to be potent motoneuron activators [PMID: 18845613].One can assume that the symptoms of narcolepsy would be treated most effectively by reversing the specific neurochemical defecits of this disorder, rather than by the administration less specific stimulants.
The two losses, of hypocretin neurons in the forebrain and of norepinephrine neurons in the brainstem locus coeruleus may be caused simultaneously or sequentially by an autoimmune process [PMID:3010426]. The HLA-DQB106:02 allele is present in 12-38% of the general population, but in 40-60% of those diagnosed with narcolepsy without cataplexy and 85-95% of those diagnosed with narcolepsy with cataplexy. A few patients having narcolepsy with cataplexy do not have the DQB106:02 allele [PMID: 21931493].
About 150,000 Americans have narcolepsy, a lifelong disorder.
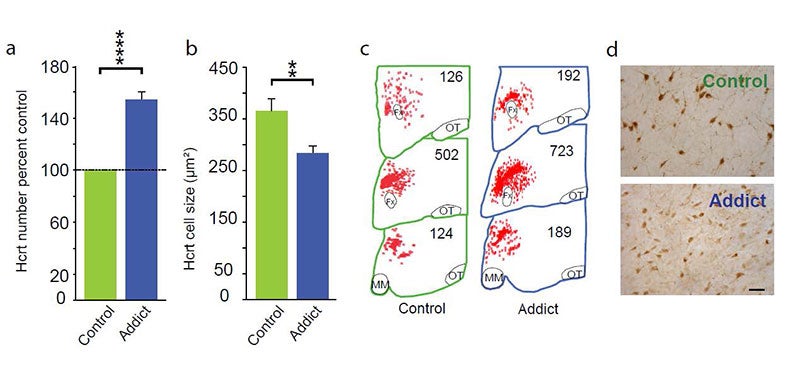
In 2018, 2024 and 2025 (below-left column on this webpage) we discovered that hypocretin neurons have a major role in opioid addiction. Chronic use of heroin in humans and daily injection of morphine in mice for 2 weeks increases the number of detected hypocretin neurons by increasing hypocretin synthesis and decreases their size (see figure below). We report that opioid dependence is prevented in mice by deleting hypocretin neurons. Humans with narcolepsy are resistant to opioid addiction [PMID: 39989723]. In 2024 we found that opioid dependence is prevented, without reducing opioid analgesia, by blocking hypocretin receptors with suvorexant when administering opioids [PMID: 39989723]. About 8 million Americans are dependent on opioids. Opioid dependence caused more than 55,000 opioid overdose deaths last year in the United States and thousands more around the world. Most individuals who die from an opioid overdose had their first dose of opioids prescribed for analgesia. Combining suvorexant with opioid administration should decrease opioid addiction and overdoses without affecting the potency of opioid analgesia. So, what started out as an inquiry into the cause of narcolepsy, a relatively rare, non lethal disease, has led us to an effective approach to reducing or preventing opioid addiction and opioid overdose death.
([PMID: 39989723], [PMID: 34853083], [PMID: 37121379]).
Video of Siegel's Keynote talk on June 9, 2025 at the Sleep 2025 meeting in Seattle. The first half discusses the function of sleep. The second half discusses the role hypocretin (orexin) and locus coeruleus neurons in narcolepsy, and the key role of hypocretin in opioid addiction.








